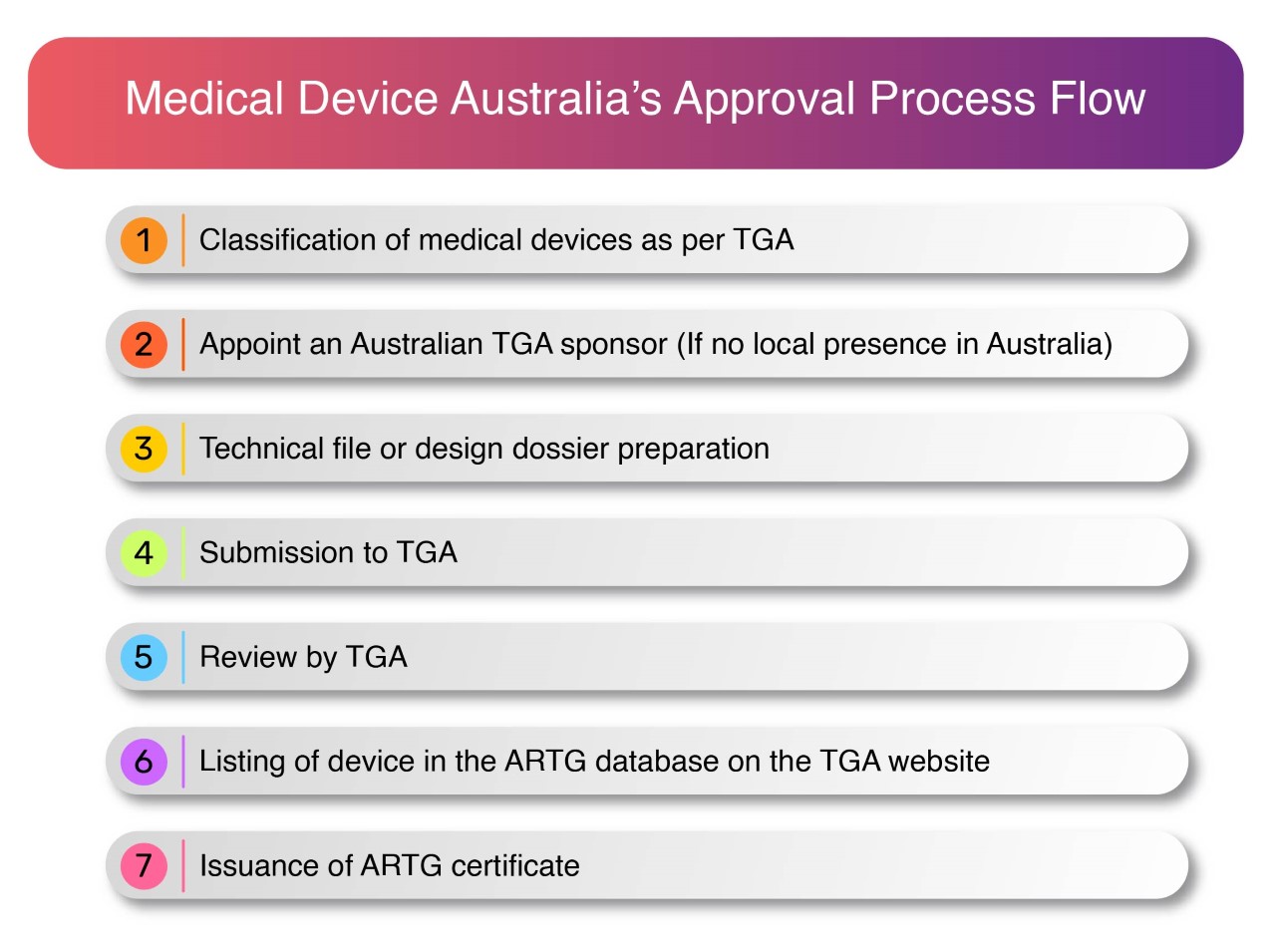
Medical Device Regulations In Australia . Clinical trial requirements for medical device registration in tga australia: — as per australian regulations, all medical devices must follow global medical device nomenclature (gmdn) guidelines. The argmd provides information on the import, export. — 3.1 medical device classifications (act s 41db).5 3.2 classification of medical devices.5 3.3. australian regulatory guidelines for medical devices (argmd) guidance. — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). — the therapeutic goods administration (tga) regulates the supply of medical devices in australia. Manufacture medical devices in australia. Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. You should report a problem or side. You may be required to comply with these guidelines if you intend to: — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. while medical devices aim to improve your health and wellbeing, it's important to know that their use also has potential risks.
from operonstrategist.com
The argmd provides information on the import, export. Manufacture medical devices in australia. — as per australian regulations, all medical devices must follow global medical device nomenclature (gmdn) guidelines. — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. You should report a problem or side. — 3.1 medical device classifications (act s 41db).5 3.2 classification of medical devices.5 3.3. — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). You may be required to comply with these guidelines if you intend to: Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. australian regulatory guidelines for medical devices (argmd) guidance.
Medical Device Registration in Australia
Medical Device Regulations In Australia — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). Clinical trial requirements for medical device registration in tga australia: Manufacture medical devices in australia. — as per australian regulations, all medical devices must follow global medical device nomenclature (gmdn) guidelines. — the therapeutic goods administration (tga) regulates the supply of medical devices in australia. — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. — 3.1 medical device classifications (act s 41db).5 3.2 classification of medical devices.5 3.3. You should report a problem or side. The argmd provides information on the import, export. Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. You may be required to comply with these guidelines if you intend to: while medical devices aim to improve your health and wellbeing, it's important to know that their use also has potential risks. — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). australian regulatory guidelines for medical devices (argmd) guidance.
From www.artixio.com
FAQs Australia (TGA) Regulations for Medical Device Registration Medical Device Regulations In Australia — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. australian regulatory guidelines for medical devices (argmd) guidance. The argmd provides information on the import, export. Clinical trial requirements for medical device registration in tga australia: — as per. Medical Device Regulations In Australia.
From www.eclevarmedtech.com
A Guide to Medical Devices Regulations Everything You Need to Know Medical Device Regulations In Australia Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. Manufacture medical devices in australia. You should report a problem or side. while medical devices aim to improve your health and wellbeing, it's important to know that their use also has potential risks. You may be required to comply with these guidelines if. Medical Device Regulations In Australia.
From www.presentationeze.com
Medical Device Instructions for Use Information & TrainingPresentationEZE Medical Device Regulations In Australia — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. Clinical trial requirements for medical device registration in tga australia: — as per australian regulations, all medical devices must follow global medical device nomenclature (gmdn) guidelines.. Medical Device Regulations In Australia.
From www.presentationeze.com
Medical Device Regulation Australia. Requirements.PresentationEZE Medical Device Regulations In Australia australian regulatory guidelines for medical devices (argmd) guidance. — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. — as per australian regulations, all medical devices must follow global medical device nomenclature (gmdn) guidelines. Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. Clinical. Medical Device Regulations In Australia.
From www.youtube.com
inar (May 2016) Medical Device Regulations in Australia YouTube Medical Device Regulations In Australia while medical devices aim to improve your health and wellbeing, it's important to know that their use also has potential risks. australian regulatory guidelines for medical devices (argmd) guidance. — the therapeutic goods administration (tga) regulates the supply of medical devices in australia. — information to assist you with australia's regulatory framework for medical devices, including. Medical Device Regulations In Australia.
From operonstrategist.com
Medical Device Registration in Australia Medical Device Regulations In Australia — 3.1 medical device classifications (act s 41db).5 3.2 classification of medical devices.5 3.3. Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. You may be required to comply with these guidelines if you intend to: — information to assist you with australia's regulatory framework for medical devices, including in vitro. Medical Device Regulations In Australia.
From commit-global.com
Medical Device Localization Regulations and Certifications Medical Device Regulations In Australia australian regulatory guidelines for medical devices (argmd) guidance. — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). — the therapeutic goods administration (tga) regulates the supply of medical devices in australia. — 3.1 medical device classifications (act s 41db).5 3.2 classification of medical devices.5 3.3. — information to assist you with australia's. Medical Device Regulations In Australia.
From www.slideshare.net
The regulation of medical devices in Australia Medical Device Regulations In Australia Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. You may be required to comply with these guidelines if you intend to: australian regulatory guidelines for medical devices (argmd) guidance. — the therapeutic goods administration (tga) regulates the supply of medical devices in australia. — collapse division 11.5—transitional provisions relating. Medical Device Regulations In Australia.
From www.presentationeze.com
Australian Medical Device regulations. PresentationEZE Medical Device Regulations In Australia — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). The argmd provides information on the import, export. You may be required to comply with these guidelines if you intend to: Clinical trial requirements for medical device registration in tga australia: — information to assist you with australia's regulatory framework for medical devices, including in vitro. Medical Device Regulations In Australia.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Regulations In Australia while medical devices aim to improve your health and wellbeing, it's important to know that their use also has potential risks. — the therapeutic goods administration (tga) regulates the supply of medical devices in australia. Manufacture medical devices in australia. The argmd provides information on the import, export. Clinical trial requirements for medical device registration in tga australia:. Medical Device Regulations In Australia.
From www.bioworld.com
Australia’s TGA delays overhaul of medical device regulations due to Medical Device Regulations In Australia — as per australian regulations, all medical devices must follow global medical device nomenclature (gmdn) guidelines. The argmd provides information on the import, export. while medical devices aim to improve your health and wellbeing, it's important to know that their use also has potential risks. australian regulatory guidelines for medical devices (argmd) guidance. Manufacture medical devices in. Medical Device Regulations In Australia.
From www.presentationeze.com
Medical Device Regulation Australia. Requirements.PresentationEZE Medical Device Regulations In Australia The argmd provides information on the import, export. Before initiating a clinical trial, researchers must obtain an investigational device exemption (ide) from the tga. Manufacture medical devices in australia. — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. — 3.1 medical device classifications (act s 41db).5 3.2 classification of medical devices.5. Medical Device Regulations In Australia.
From www.slideshare.net
Australia medical device approval chart Emergo Group Medical Device Regulations In Australia — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). Manufacture medical devices in australia. — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. You should report a problem or side. Clinical trial requirements for medical device registration in tga australia: — 3.1 medical device classifications (act s. Medical Device Regulations In Australia.
From www.slideserve.com
PPT The regulation of medical devices in Australia PowerPoint Medical Device Regulations In Australia The argmd provides information on the import, export. — the therapeutic goods administration (tga) regulates the supply of medical devices in australia. You may be required to comply with these guidelines if you intend to: — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). — 3.1 medical device classifications (act s 41db).5 3.2 classification. Medical Device Regulations In Australia.
From www.scribd.com
Australian Regulatory Guidelines for Medical Devices Medical Device Medical Device Regulations In Australia Manufacture medical devices in australia. while medical devices aim to improve your health and wellbeing, it's important to know that their use also has potential risks. — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. You should report a problem or side. — the therapeutic goods administration (tga) regulates the. Medical Device Regulations In Australia.
From www.regdesk.co
Australia Medical Device Regulations RegDesk Medical Device Regulations In Australia australian regulatory guidelines for medical devices (argmd) guidance. — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). Manufacture medical devices in australia. Clinical trial requirements for medical device registration in tga australia: — 3.1 medical device classifications (act s 41db).5 3.2 classification of medical devices.5 3.3. — the therapeutic goods administration (tga) regulates. Medical Device Regulations In Australia.
From www.slideshare.net
The regulation of medical devices in Australia Medical Device Regulations In Australia — the therapeutic goods administration (tga) regulates the supply of medical devices in australia. — collapse division 11.5—transitional provisions relating to the therapeutic goods (medical devices). Manufacture medical devices in australia. — information to assist you with australia's regulatory framework for medical devices, including in vitro diagnostics. — 3.1 medical device classifications (act s 41db).5 3.2. Medical Device Regulations In Australia.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Medical Device Regulations In Australia Manufacture medical devices in australia. — 3.1 medical device classifications (act s 41db).5 3.2 classification of medical devices.5 3.3. The argmd provides information on the import, export. You may be required to comply with these guidelines if you intend to: — as per australian regulations, all medical devices must follow global medical device nomenclature (gmdn) guidelines. Clinical trial. Medical Device Regulations In Australia.